

 |
zurück |  |
3. Literaturangaben
(1) Kukowski H. and Brümmer G., (1987). Investigations on the Adsorption and Desorption of Selected Chemicals in Soils. UBa Report 106 02 045, Part II.
(2) Fränzle O., Kuhnt G. and Vetter L., (1987). Selection of Representative Soils in the EC-Territory. UBa Report 106 02 045, Part I.
(3) Kuhnt G. and Muntau H. (Eds.) EURO-Soils: Identification, Collection, Treatment, Characterisation. Special Publication No 1.94.60, Joint Research Centre. European Commission, ISPRA, December 1994.
(4) OECD Test Guidelines Programme, Final Report of the OECD Workshop on Selection of Soils/Sediments, Belgirate, Italy, 18-20 January 1995 (June 1995).
(5) US Environment Protection Agency: Pesticide Assessment Guidelines, Subdivision N, Chemistry: Environmental Fate, Series 163-1, Leaching and Adsorption/Desorption Studies, Addendum 6 on Data Reporting, 540/09-88-096, Date: 1/1988.
(6) US Environment Protection Agency: Prevention, Pesticides and Toxic Substances, OPPTS Harmonized Test Guidelines, Series 835-Fate, Transport and Transformation Test Guidelines, 0PPTS No: 835.1220 Sediment and Soil Adsorption/Desorption Isotherm. EPa No: 712-C-96-048, April 1996.
(7) ASTM Standards, E 1195-85, Standard Test Method for Determining a Sorption Constant (Koc) for an Organic Chemical in Soil and Sediments.
(8) Agriculture Canada: Environmental Chemistry and Fate. Guidelines for registration of pesticides in Canada, 15 July 1987.
(9) Netherlands Commission Registration Pesticides (1995): Application for registration of a pesticide. Section G. Behaviour of the product and its metabolites in soil, water and air.
(10) Danish National Agency of Environmental Protection (October 1988): Criteria for registration of pesticides as especially dangerous to health or especially harmful to the environment.
(11) BBa (1990), Guidelines for the Official Testing of Plant Protection Products, Biological Research Centre for Agriculture and Forestry, Braunschweig, Germany.
(12) Calvet R., (1989), "Evaluation of adsorption coefficients and the prediction of the mobilities of pesticides in soils", in Methodological Aspects of the Study of Pesticide Behaviour in Soil (ed. P. Jamet), INRA, Paris, (Review).
(13) Calvet R., (1980), "Adsorption-Desorption Phenomena" in Interactions between herbicides and the soil. (R. J. Hance ed.), Academic Press, London, pp. 83-122.
(14) Hasset J. J., and Banwart W.L., (1989), "The sorption of nonpolar organics by soils and sediments" in Reactions and Movement of Organic Chemicals in Soils. Soil Science Society of America (S.S.S.A), Special Publication no. 22, pp. 31-44.
(15) van Genuchten M. Th., Davidson J. M., and Wierenga P. J., (1974), "An evaluation of kinetic and equilibrium equations for the prediction of pesticide movement through porous media". Soil Sci. Soc. Am. Proc., Vol. 38(1), pp. 29-35.
(16) McCall P. J., Laskowski D. A., Swann R. L., and Dishburger H. J., (1981), "Measurement of sorption coefficients of organic chemicals and their use, in environmental fate analysis", in Test Protocols for Environmental Fate and Movement of Toxicants. Proceedings of AOAC Symposium, AOAC, Washington DC.
(17) Lambert S. M., Porter P. E. and Schieferrstein R. H., (1965), "Movement and sorption of chemicals applied to the soil". Weeds, 13, pp. 185-190.
(18) Rhodes R. C., Belasco I. J., and Pease H. L., (1970) "Determination of mobility and adsorption of agrochemicals in soils". J.Agric.Food Chem., 18, pp. 524-528.
(19) Russell M. H., (1995), "Recommended approaches to assess pesticide mobility in soil" in Environmental Behavior of Agrochemicals (ed. T. R. Roberts and P. C. Kearney). John Wiley & Sons Ltd.
(20) Esser H. O., Hemingway R. J., Klein W., Sharp D. B., Vonk J. W. and Holland P. T., (1988), "Recommended approach to the evaluation of the environmental behavior of pesticides", IUPAC Reports on Pesticides (24). Pure Appl. Chem., 60, pp. 901-932.
(21) Guth J. A., Burkhard N., and D. O. Eberle, (1976), "Experimental models for studying the persistence of pesticides in soils". Proc. BCPC Symposium: Persistence of Insecticides and Herbicides, pp. 137-157, BCPC, Surrey, UK.
(22) Furminge C. G. L., and Osgerby J. M., (1967), "Persistence of herbicides in soil". J. Sci. Fd Agric., 18, pp. 269-273.
(23) Burkhard N., and Guth J. A., (1981), "Chemical hydrolysis of 2-Chloro-4,6-bis(alkylamino)-1,3,5-triazine herbicides and their breakdown in soil under the influence of adsorption". Pestic. Sci. 12, pp. 45-52.
(24) Guth J. A., Gerber H. R., and Schlaepfer T., (1977), "Effect of adsorption, movement and persistence on the biological availability of soil-applied pesticides". Proc. Br. Crop Prot. Conf., 3, pp. 961-971.
(25) Osgerby J. M., (1973), "Process affecting herbicide action in soil". Pestic. Sci., 4, pp. 247-258.
(26) Guth J. A., (1972), "Adsorptions- und Einwascheverhalten von Pflanzenschutzmitteln in Böden". Schr. Reihe Ver. Wass. -Boden-Lufthyg. Berlin-Dahlem, Heft 37, pp. 143-154.
(27) Hamaker J. W., (1975), "The interpretation of soil leaching experiments" in Environmental Dynamics of Pesticides (eds R. Haque and V.H. freed), pp. 135-172, Plenum Press, NY.
(28) Helling C. S., (1971), "Pesticide mobility in soils" Soil Sci. Soc. Amer. Proc., 35, pp. 732-210.
(29) Hamaker J. W., (1972), "Diffusion and volatilization" in Organic chemicals in the soil environment (C.A.I. Goring and J. W. Hamaker eds), Vol. I, pp. 49-143.
30) Burkhard N. and Guth J. A., (1981), "Rate of volatilisation of pesticides from soil surfaces; Comparison of calculated results with those determined in a laboratory model system". Pestic. Sci. 12, pp. 37-44.
(31) Cohen S. Z., Creeger S. M., Carsel R.F., and Enfield C.G., (1984), "Potential pesticide contamination of groundwater from agricultural uses", in Treatment and Disposal of Pesticide Wastes, pp. 297-325, Acs Symp. Ser. 259, American Chemical Society, Washington, DC.
(32) Gustafson D. I., (1989), "Groundwater ubiquity score: a simple method for assessing pesticide leachability". J. Environ. Toxic. Chem., 8(4), pp. 339-357.
(33) Leistra M., and Dekkers W. A., (1976). "Computed effects of adsorption kinetics on pesticide movement in soils". J. of Soil Sci., 28, pp. 340-350.
(34) Bromilov R. H., and Leistra M., (1980), "Measured and simulated behavior of aldicarb and its oxydation products in fallow soils". Pest. Sci., 11, pp. 389-395.
(35) Green R. E., and Karickoff S. W., (1990), "Sorption estimates for modeling", in Pesticides in the Soil Environment: Process, Impacts and Modeling (ed. H.H. Cheng). Soil Sci. Soc. Am., Book Series no. 2, pp. 80-101,
(36) Lambert S. M., (1967), "Functional relationship between sorption in soil and chemical structure". J. Agri. Food Chem., 15, pp. 572-576.
(37) Hance R. J., (1969), "An empirical relationship between chemical structure and the sorption of some herbicides by soils". J. Agri. Food Chem., 17, pp. 667-668.
(38) Briggs G. G. (1969), "Molecular structure of herbicides and their sorption by soils". Nature, 223, 1288.
(39) Briggs G. G. (1981). "Theoretical and experimental relationships between soil adsorption, octanol-water partition coefficients, water solubilities, bioconcentration factors, and the parachor". J. Agric. Food Chem., 29, pp. 1050-1059.
(40) Sabljic A., (1984), "Predictions of the nature and strength of soil sorption of organic polutance by molecular topology". J. Agric. Food Chem., 32, pp. 243-246.
(41) Bailey G. W., and White J. L., (1970), "Factors influencing the adsorption, desorption, and movement of pesticides in soil". Residue Rev., 32, pp. 29-92.
(42) Bailey G. W., J. L. White and Y. Rothberg., (1968), "Adsorption of organic herbicides by montomorillonite: Role of pH and chemical character of adsorbate". Soil Sci. Soc. Amer. Proc. 32: pp. 222-234.
(43) Karickhoff S. W., (1981), "Semi-empirical estimation of sorption of hydrophobic pollutants on natural sediments and soils". Chemosphere 10, pp. 833-846.
(44) Paya-Perez A., Riaz M. and Larsen B., (1989), "Soil Sorption of 6 Chlorobenzenes and 20 PCB Congeners". Environ. Toxicol. Safety 21, pp. 1-17.
(45) Hamaker J. W., and Thompson J. M., (1972), "Adsorption in organic chemicals" in Organic Chemicals in the Soil Environment (Goring C.A.I. and Hamaker J.W., eds), Vol I and II, Marcel Dekker, Inc., New York, NY, 1972, pp. 49-143.
(46) Deli J., and Warren G. F., 1971, "Adsorption, desorption and leaching of diphenamid in soils". Weed Sci. 19: pp. 67-69.
(47) Chu-Huang Wu, Buehring N., Davinson J. M. and Santelmann, (1975), "Napropamide Adsorption, desorption and Movement in soils". Weed Science, Vol. 23, pp. 454-457.
(48) Haues M. H. B., Stacey M., and Thompson J. M., (1968), "Adsorption of s-triazine herbicides by soil organic preparations" in Isotopes and Radiation in Soil Organic Studies, p.75, International. Atomic Energy Agency, Vienna.
(49) Pionke H. B., and Deangelis R. J., (1980), "Methods for distributing pesticide loss in field run-off between the solution and adsorbed phase", CREAMS, in a Field Scale Model for Chemicals, Run-off and Erosion from Agricultural Management Systems, Chapter 19, Vol. III: Supporting Documentation, USDa Conservation Research report.
(50) ISO Standard Compendium Environment: Soil Quality - General aspects; chemical and physical methods of analysis; biological methods of analysis. First Edition (1994).
(51) Scheffer F., and Schachtschabel, Lehrbuch der Bodenkunde, F. Enke Verlag, Stuttgart (1982), 11th edition.
(52) Black, Evans D. D., White J. L., Ensminger L. E., and Clark F. E., eds. "Methods of Soil Analysis", Vol 1 and 2, American Society of Agronomy, Madison, WI, 1982.
(53) ISO/DIS 10381-1 Soil Quality - Sampling - Part 1: Guidance on the design of sampling programmes.
(54) ISO/DIS 10381-2 Soil Quality - Sampling - Part 2: Guidance on sampling techniques.
(55) ISO/DIS 10381-3 Soil Quality - Sampling - Part 3: Guidance on safety of sampling.
(56) ISO/DIS 10381-4 Soil Quality - Sampling - Part 4: Guidance on the investigation of natural and cultivated soils.
(57) ISO/DIS 10381-5 Soil Quality - Sampling - Part 5: Guidance on the investigation of soil contamination of urban and industrial sites.
(58) ISO 10381-6, 1993: Soil Quality - Sampling - Part 6: Guidance on the collection, handling and storage of soil for the assessment of aerobic microbial processes in the laboratory.
(59) Green R. E., and Yamane V. K., (1970), "Precision in pesticide adsorption measurements". Soil Sci. Am. Proc., 34, pp. 353-354.
(60) Grover R., and Hance R. J. (1970), "Effect of ratio of soil to water on adsorption of linuron and atrazine". Soil Sci., pp. 109-138.
(61) Boesten, J. J. T. I, "Influence of soil/liquid ratio on the experimental error of sorption coefficients in pesticide/soil system". Pest. Sci. 1990, 30, pp. 31-41.
(62) Boesten, J. J. T. I. "Influence of soil/liquid ratio on the experimental error of sorption coefficients in relation to OECD guideline 106". Proceedings of 5th international workshop on environmental behaviour of pesticides and regulatory aspects, Brussels, 26-29 April 1994.
(63) Bastide J., Cantier J. M., et Coste C., (1980), "Comportement de substances herbicides dans le sol en fonction de leur structure chimique". Weed Res. 21, pp. 227-231.
(64) Brown D. S., and Flagg E. W., (1981), "Empirical prediction of organic pollutants sorption in natural sediments". J. Environ.Qual., 10(3), pp. 382-386.
(65) Chiou C. T., Porter P. E., and Schmedding D. W., (1983), "Partition equilibria of non-ionic organic compounds between soil organic matter and water". Environ. Sci. Technol., 17(4), pp. 227-231.
(66) Gerstl Z., and Mingelgrin U., (1984), "Sorption of organic substances by soils and sediments". J. Environm. Sci. Health, B19 (3), pp. 297-312.
(67) Vowles P. D., and Mantoura R. F. C., (1987), "Sediment-water partition coefficient and HPLC retention factors of aromatic hydrocarbons". Chemosphere, 16(1), pp. 109-116.
(68) Lyman W. J. , Reehl W. F.and Rosenblatt D. H. (1990). Handbook of Chemical Property Estimation Methods. Environmental Behaviour of Organic Compounds. American Chemical Society, Washington DC.
(69) Keniga E. E., and Goring, C. A. I. (1980). "Relationship between water solubility, soil sorption, octanol-water partitioning and concentration of chemicals in the biota" in Aquatic Toxicology (eds J.G. Eaton, et al.), pp.78-115, ASTM STP 707, Philadelphia.
(70) Chiou C. T., Peters L. J., and Freed V. H., (1979), "a physical concept of soil-water equilibria for non-ionic organic compounds". Science, Vol. 206, pp. 831-832.
(71) Hassett J. J., Banwart W. I., Wood S. G., and Means J. C., (1981), "Sorption of /-Naphtol: implications concerning the limits of hydrophobic sorption". Soil Sci. Soc. Am. J. 45, pp. 38-42.
(72) Karickhoff S. W., (1981), "Semi-empirical estimation of sorption of hydrophobic pollutants on natural sediments and soils" Chemosphere, Vol. 10(8), pp. 833-846.
(73) Moreale A., van Bladel R., (1981), "Adsorption de 13 herbicides et insecticides par le sol. Relation solubilité-reactivité". Revue de l'Agric., 34 (4), pp. 319-322.
(74) Müller M., Kördel W. (1996), "Comparison of screening methods for the determination/estimation of adsorption coefficients on soil". Chemosphere, 32(12), pp. 2493-2504.
(75) Kördel W., Kotthoff G., Müller M. (1995), "HPLC - screening method for the determination of the adsorption coefficient on soil - results of a ring test". Chemosphere 30 (7), pp. 1373-1384.
(76) Kördel W., Stutte J., Kotthoff G. (1993), "HPLC - screening method for the determination of the adsorption coefficient on soil - comparison of different stationary phases" Chemosphere 27 (12), pp. 2341-2352.
(77) Hance, R. J., (1967), "The Speed of Attainment of Sorption Equilibria in Some Systems Involving Herbicides". Weed Research, Vol. 7, pp. 29-36.
(78) Koskinen W. C., and Harper S., (1990), "The retention processes: mechanisms" in Pesticides in the Soil Environment: Processes, Impacts and Modelling (ed. H.H. Cheng). Soil Sci. Soc. Am. Book Series, No. 2, Madison, Wisconsin.
(79) Cohen S. Z., Creeger S. M., Carsel R. F., and Enfield C. G. (1984), "Potential pesticide contamination of groundwater from agricultural uses", in Treatment and Disposal of Pesticide Wastes, pp.297-325, ACS Symp. Ser. 259, American Chemical Society, Washington, DC.
(80) Giles C. H., (1970), "Interpretation and use of sorption isotherms" in Sorption and Transport Processes in Soils. S.C.I. Monograph No. 37, pp. 14-32.
(81) Giles, C. H.; McEwan J. H.; Nakhwa, S.N. and Smith, D, (1960), "Studies in adsorption: XI. a system of classification of solution adsorption isotherms and its use in the diagnosis of adsorption mechanisms and in measurements of pesticides surface areas of soils". J. Chem. Soc., pp. 3973-93.
(82) Calvet R., Tercé M., and Arvien J. C., (1980), "Adsorption des pesticides par les sols et leurs constituants: 3. Caractéristiques générales de l'adsorption". Ann. Agron. 31: pp. 239-251.
(83) Bedbur E., (1996), "Anomalies in the Freundlich equation", Proc. COST 66 Workshop, Pesticides in soil and the environment, 13-15 May 1996, Stratford-upon-Avon, UK.
(84) Guth, J. A., (1985), "Adsorption/desorption", in Joint International Symposium, Physicochemical Properties and their Role in Environmental Hazard Assessment, July 1-3, Canterbury, UK.
(85) Soil Texture Classification (US and FAO systems): Weed Science, 33, Suppl. 1 (1985) and Soil Sci. Soc. Amer. Proc. 26:305 (1962).
| Bestimmung der Ökotoxizität
C.18. Adsorption/Desorption nach einer Schüttelmethode |
Anhang V C.18. zur RL 67/548/EWG Anlage 1 |
| Bestimmung der Ökotoxizität
C.18. Adsorption/Desorption nach einer Schüttelmethode Einfluss der Genauigkeit der Analysenmethode und der Konzentrationsveränderung auf die Genauigkeit von Adsorptionsergebnissen |
Anhang V C.18. zur RL 67/548/EWG Anlage 2 |
Wie die nachstehende Tabelle (84) verdeutlicht, führt dann, wenn die Differenz zwischen der Anfangsmasse (m0 = 110 µg) und der Gleichgewichtsmasse (maqads (eq) = 100 µg) der Testsubstanz sehr gering ist, eine Abweichung von 5 % bei der Messung der Gleichgewichtskonzentration zu einer Abweichung von 50 % bei der Berechnung des Gehalts der an Boden adsorbierten Substanz (msads (eq)) und von 52,4 % bei der Berechnung von Kd.
| Menge des Bodens | mBoden | = 10 g |
| Volumen der Lösung | V0 | = 100 cm3 |
| maqads (eq) (µg) |
Caqads (eq) (µg cm-3) |
R | (msads (eq)* (µg) |
Csads (eq)* (µg g-1 ) |
Rx | Kd * | Rx |
| m0 = 110 µg oder C0 = 1100 µg/cm3 µg
FÜR a = 9 % |
|||||||
| 100 | 1,000 | wahrer Wert | 10 | 1,00 | wahrer Wert | 1 | |
| 101 | 1,010 | 1 % | 9 | 0,90 | 10 % | 0,891 | 10,9 % |
| 105 | 1,050 | 5 % | 5 | 0,50 | 50 % | 0,476 | 52,4 % |
| 109 | 1,090 | 9 % | 1 | 0,10 | 90 % | 0,092 | 90,8 % |
| m0 = 110 µg oder C0 = 1100 µg/cm3 µg
FÜR a = 55 % |
|||||||
| 50,0 | 0,500 | wahrer Wert | 60,0 | 6,00 | wahrer Wert | 12,00 | |
| 50,5 | 0,505 | 1 % | 59,5 | 5,95 | 0,8 % | 11,78 | 1,8 % |
| 52,5 | 0,525 | 5 % | 57,5 | 5,75 | 4,0 % | 10,95 | 8,8 % |
| 55,0 | 0,550 | 10 % | 55,0 | 5,50 | 8,3 % | 10,00 | 16,7 % |
| m0 = 110 µg oder C0 = 1100 µg/cm3 µg
FÜR a = 99 % |
|||||||
| 1,100 | 0,011 | wahrer Wert | 108,9 | 10,89 | wahrer Wert | 990 | |
| 1,111 | 0,01111 | 1 % | 108,889 | 10,8889 | 0,01 % | 980 | 1,0 % |
| 1,155 | 0,01155 | 5 % | 108,845 | 10,8845 | 0,05 % | 942 4, | 8 % |
| 1,21 | 0,0121 | 10 % | 108,790 | 10,8790 | 0,10 % | 899 | 9,2 % |
Hierin bedeuten:
| *msads(eq) | = | 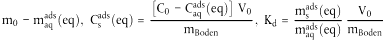 |
| msads (eq) | = | Masse der Testsubstanz in der Bodenphase bei Gleichgewicht, µg; |
| maqads(eq) | = | Masse der Testsubstanz in der wässrigen Phase bei Gleichgewicht, µg; |
| Csads (eq) | = | Gehalt der Testsubstanz in der Bodenphase bei Gleichgewicht, µg g-1; |
| Caqads (eq) | = | Massenkonzentration der Testsubstanz in der wässrigen Phase bei Gleichgewicht, µg cm-3; |
| R | = | Analysenfehler bei der Bestimmung der maqads (eq). |
| Rx | = | rechnerische Abweichung als Folge des Analysenfehlers R. |
| Bestimmung der Ökotoxizität C.18. Adsorption/Desorption nach einer Schüttelmethode Abschätzungsverfahren für Kd |
Anhang V zur RL 67/548/EWG Anlage 3 |
| 100 | Kd | 100 | ||||||
| Koc = Kd | ( cm 3 g-1) | Kom = | · | ( cm 3 g-1) | ||||
| %oc | 1,724 | %oc |
Tabelle 1. Beispielhafte Korrelationen zwischen dem Adsorptionsverteilungskoeffizienten und dem Octanol-WasserVerteilungskoeffizienten; weitere Beispiele siehe (12) (68).
| Substanzen | Korrelationen | Verfasser |
| Substituierte Harnstoffe | log Kom = 0,69 + 0,52 log Pow | Briggs (1981) (39) |
| Aromatische chlorierte Substanzen | log Koc= -0,779 + 0,904 log Pow | Chiou et al. (1983) (65) |
| Verschiedene Pestizide | log Kom= 4,4 + 0,72 log Pow | Gerstl und Mingelgrin (1984) (66) |
| Aromatische Kohlenwasserstoffe | log Koc = -2,53 + 1,15 log Pow | Vowles und Mantoura (1987) (67) |
Tabelle 2. Beispielhafte Korrelationen zwischen dem Adsorptionsverteilungskoeffizienten und der Wasserlöslichkeit; weitere Beispiele siehe (68) (69).
| Verbindungen | Korrelationen | Verfasser |
| Verschiedene Pestizide | log Kom = 3,8 - 0,561 log Sw | Gerstl und Mingelgrin (1984) (66) |
| Aliphatische, aromatische chlorierte Substanzen | log Kom = (4,040 +/- 0,038) - (0.557 +/- 0.012) log Sw | Chiou et al. (1979) (70) |
| α -Naphtol | log Koc = 4,273 - 0,686 log Sw | Hasset et al. (1981) (71) |
| Cyclische, aliphatische aromatische Substanzen | log Koc = -1,405 - 0,921 log Sw - 0,00953 (mp-25) | Karickhoff (1981) (72) |
| Verschiedene Verbindungen | log Kom = 2,75 - 0,45 log Sw | Moreale van Blade (1982) (73) |
 |
weiter . |  |
(Stand: 04.08.2022)
Alle vollständigen Texte in der aktuellen Fassung im Jahresabonnement
Nutzungsgebühr: ab 105.- € netto
(derzeit ca. 7200 Titel s.Übersicht - keine Unterteilung in Fachbereiche)
Die Zugangskennung wird kurzfristig übermittelt
? Fragen ?
Abonnentenzugang/Volltextversion